PVA polymer slime

This experiment is easy to set up, providing the chemicals are available, and should take no more than about 30 minutes. It can be done by students in groups of two or three.
Related resources
- An alternative method aimed at 11–12 years and designed for outreach is offered in the snot section of our Snap, crackle and snot resource.
- Also designed for outreach, Year 9 chemistry day shows how to make bouncing custard.
Equipment
Apparatus
- Saftefy glasses
- Beaker (100 cm 3 )
- Measuring cylinder (50 cm 3 )
- Disposable plastic cup
- Metal spatula
- Petri dish (or watch glass)
- Water-based felt-tipped pen
- Spirit-based felt-tipped pen
- Disposable plastic gloves
Chemicals
- PVA, polyvinyl alcohol, 4% (or 8%) aqueous solution, 40 cm 3 (see note 3 below)
- Borax, hydrated sodium tetraborate, 4% (or 8%) aqueous solution, 10 cm 3
- Food colour or fluorescein (optional)
- Hydrochloric acid, about 0.5 M, 20 cm 3 (optional)
- Sodium hydroxide, 0.4 M (IRRITANT), 20 cm 3 (optional)
Health, safety and technical notes
- Read our standard health and safety guidance
- Wear eye protection, and protective gloves if handling the slime.
- Polyvinyl alcohol, (-[CH2CH(OH)]n-) – PVA can be high molecular weight (MW), about 120 000, or low MW, about 15 000. If high MW PVA is used, prepare a 4% solution by placing 960 cm 3 of water into a tall 1 dm 3 beaker. Measure out 40 g of high MW PVA and add this slowly to the beaker of water, with stirring. If low MW PVA is used, prepare an 8% solution by placing 920 cm 3 of water into a tall 1 dm 3 beaker. Measure out 80 g of low MW PVA and add this slowly to the beaker of water, with stirring. In each case, heat the mixture gently, stirring occasionally, until the solution clears. Avoid boiling the solution. After cooling, this solution can be poured into suitable smaller containers, which can then be sealed and stored indefinitely.
- Borax, hydrated sodium tetraborate, (Na2B4O7.10H2O) – see CLEAPSS Hazcard HC014a. If a 4% aqueous solution of PVA is used a 4% aqueous solution of borax will be required. If an 8% aqueous solution of PVA is used an 8% aqueous solution of borax will be required. Borax solid is now classified as TOXIC because it carries the R60/61 warning – it may cause infertility and harm to the unborn child. The solid and solutions in excess of 8.5% should be labelled TOXIC, but other solutions will NOT carry any hazard warning. Borax solutions as prepared following these instructions do NOT require a hazard label. BUT technicians preparing the solutions need to be alerted to the hazard. CLEAPSS advises weighing the solid very carefully, possibly in a fume cupboard which is not switched on. Pregnant women should avoid handling the solid. Students may want to take the slime home – this should not be allowed.
- Fluorescein – see CLEAPSS Hazcard HC032 and CLEAPSS Recipe Book RB000.
- Hydrochloric acid, HCl(aq) – see CLEAPSS Hazcard HC047a and CLEAPSS Recipe Book RB043. Best supplied in small glass bottles fitted with teat pipettes.
- Sodium hydroxide, NaOH(aq), (CORROSIVE) – see CLEAPSS Hazcard HC091a and CLEAPSS Recipe Book RB085. Best supplied in small glass bottles fitted with teat pipettes.
- Slime – see CLEAPSS Recipe Book RB000. As per note 4, the slime is NOT TOXIC but students should not be allowed to take the slime home.
Procedure
- Place 40 cm 3 of the polyvinyl alcohol solution in the plastic cup.
- If supplied, add one drop of food colour or fluorescein dye to the solution. Stir well.
- Measure out 10 cm 3 of borax solution into the beaker and add this to the polyvinyl alcohol solution, stirring vigorously until gelling is complete. This gel is sometimes known as ‘slime’.
- Wearing disposable gloves, remove the slime from the cup and knead it thoroughly to mix the contents completely. Roll the slime around in your hand, gently squeezing the material to remove air bubbles at the same time. Alternatively, place the slime in a plastic bag and mix and squeeze the mixture from outside the bag.
Show Fullscreen
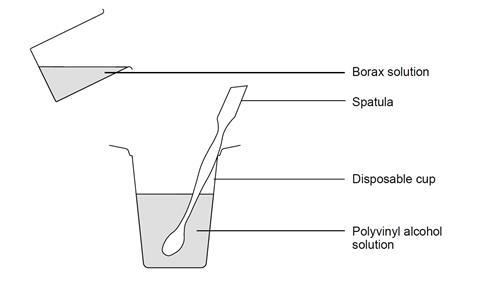
Source: Royal Society of Chemistry
The apparatus set-up for making slime out of PVA and borax solution
Test the properties of your slime in the following ways (tests 6–8 below are optional):
- Pull the slime apart slowly. What happens?
- Pull the slime apart sharply and quickly. What happens?
- Roll the slime into a ball and drop it on to the bench. What happens?
- Place a small bit of slime on the bench and hit it hard with your hand. What happens?
- Write your name on a piece of paper with a water-based felt-tipped pen. Place the slime on top, press firmly, and then lift up the slime. What has happened to the writing and to the slime? Try the same again, this time using a spirit-based pen. Does this show the same effect?
- Place a very small piece of slime in a Petri dish. Add the dilute hydrochloric acid dropwise, stirring well after each drop. When you notice a change, record the number of drops added and your observations.
- Now add dilute sodium hydroxide solution to the same sample used above in 6, stirring after each drop. When you notice a change record the number of drops added and your observations.
- Can tests 6 and 7 be repeated time and time again to give the same results?
Teaching notes
Tell students to keep the slime away from clothes as it can produce permanent stains. The slime can be stored in an air-tight container, such as a plastic bag with a twist-tie. It is advisable to dip the slime in some water before storing, to keep it from drying out. Slime gets dirty from handling and may become mouldy after several days. When this happens you should throw it away. Do not put it down the sink because it clogs the drain.
Slime-type materials are available under a variety of different brand names, and can be found in many toy stores. Slime is sometimes described as a reversible cross-linking gel. The cross-linking between the polymer chains of polyvinyl alcohol occurs by adding borax, Na2B4O7.10H2O (sodium tetraborate).
PVA glue contains, amongst other things, the polymer polyvinyl alcohol (also called polyethenol). Polyvinyl alcohol has the structure:
Show Fullscreen
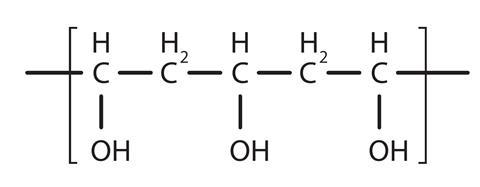
Source: Royal Society of Chemistry
The structure of polymer polyvinyl alcohol (also called polyethenol), which is contained in PVA glue.
Borax forms the borate ion when in solution. This ion has the structure:
Show Fullscreen
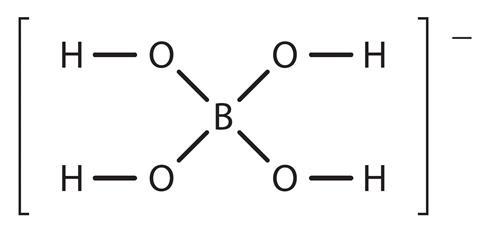
Source: Royal Society of Chemistry
The structure of the borate ion, used in making slime out of PVA.
The borate ion can make weak bonds with the OH groups in the polymer chains so it can link the chains together as shown below. This is called cross-linking:
Show Fullscreen
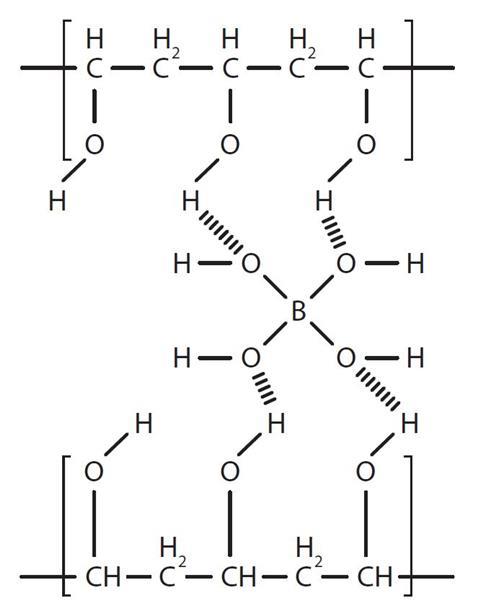
Source: Royal Society of Chemistry
Cross-linking between borate ions and the OH groups in the polymer chains of PVA
Slime is a non-Newtonian fluid that is dilatant, ie under stress, the material dilates or expands. Other well known stress-thickening materials are quicksand, wet sand on the beach, some printer’s inks, starch solutions and ‘Silly Putty’. Dilatant materials tend to have some unusual properties:
- Under low stress, such as slowly pulling on the material, it will flow and stretch. If careful, you can form a thin film.
- Pull sharply (high stress) and the material breaks.
- Pour the material from its container then tip the container upwards slightly, the gel self siphons.
- Put a small amount of the material on a table top and hit it with your hand, there is no splashing or splattering.
- Throw a small piece onto a hard surface; it will bounce slightly.
- Adding acid to the slime breaks the crosslinking producing a liquid with lower viscosity. Adding alkali reverses the process and the slime should be regenerated.
Various types of slime have been manufactured. In this investigation you use the polymer polyvinyl alcohol, which is reasonably cheap and is readily available from suppliers because it is widely used as a thickener, stabiliser and binder in cosmetics, paper cloth, films, cements and mortars. In ethanol solution polyvinyl alcohol solution dries to leave a thin plastic film that is useful in packaging materials, especially as it is biodegradable. PVA glue can be partially or completely hydrolysed to give polyvinyl alcohol.
For further information on slime, visit how products are made.
More resources
Add context and inspire your learners with our short career videos showing how chemistry is making a difference.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry






